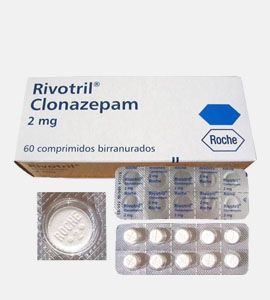
Klonopin (Clonazepam)
* Alleen ter illustratie
Klonopin (Clonazepam)
Korte omschrijving
Klonopin (Clonazepam) 2mg (UK) 30pillen 30 pillen
93,528.83 HUF
Klonopin (Clonazepam) 2mg (UK) 60pillen 60 pillen
136,695.98 HUF
Klonopin (Clonazepam) 2mg (UK) 90pillen 90 pillen
179,484.47 HUF
Klonopin (Clonazepam) 2mg (UK) 120pillen 120 pillen
222,651.62 HUF
Klonopin (Clonazepam) 2mg (UK) 180pillen 180 pillen
308,985.92 HUF
Klonopin (Clonazepam) 2mg (UK) 240pillen 240 pillen
394,941.57 HUF
Klonopin (Clonazepam) 2mg (UK) 270pillen 270 pillen
438,108.72 HUF
Klonopin (Clonazepam) 2mg (UK) 360pillen 360 pillen
567,231.51 HUF
Klonopin (Clonazepam) 2mg (EU) 30pillen 30 pillen
70,051.96 HUF
Populair Klonopin (Clonazepam) 2mg (EU) 60pillen 60 pillen
95,422.12 HUF
Klonopin (Clonazepam) 2mg (EU) 90pillen 90 pillen
120,792.29 HUF
Klonopin (Clonazepam) 2mg (EU) 120pillen 120 pillen
146,162.46 HUF
Klonopin (Clonazepam) 2mg (EU) 180pillen 180 pillen
197,281.45 HUF
Klonopin (Clonazepam) 2mg (EU) 240pillen 240 pillen
248,021.79 HUF
Klonopin (Clonazepam) 2mg (EU) 270pillen 270 pillen
273,391.96 HUF
Klonopin (Clonazepam) 2mg (EU) 360pillen 360 pillen
349,502.46 HUF
toegevoegd aan winkelwagentje
-
Verzending5-7 dagenGegarandeerde levertijd 16 dagenGegarandeerde levertijd 16 dagen
-
Betaling






-
Upgrade en bespaar. Voeg items aan je winkelwagentje toe en krijg gratis verzending
75731.84 HUF




Klonopin (Clonazepam)
Regarding Shipping: Products with dosages mentioned (EU), are shipped inside Europe and garantuee faster time in delivery.